1 boiler desulphurisation wastewater causes and the importance of treatment
Wastewater generated from the process of wet desulphurisation of boiler flue gas (limestone/gypsum method) mainly comes from gypsum dewatering and cleaning system absorption tower discharge water, which is generated and discharged from the system in order to maintain the balance of substances in the slurry circulation system of the desulphurisation plant, prevent the soluble part of flue gas, i.e., the concentration of chlorine, from exceeding the prescribed value and to ensure the quality of gypsum. In order to meet the national secondary emission standards, heavy metals, suspended solids, etc., need to be removed from the wastewater; and its pH value needs to be adjusted to a suitable range. The national secondary discharge standard and typical FGD wastewater comparison indicators are shown in Table 1.
2 Desulphurisation wastewater treatment process principle
The impurities contained in the wastewater mainly include suspended solids, oversaturated sulfites, sulfates and heavy metals. The main characteristics of the popular wet desulfurisation wastewater at this stage are: (1) The wastewater is weakly acidic, with a low pH value; the suspended solids are high, but the particles are fine, and the main components are dust and desulfurisation products (CaSO4 and CaSO3). (2) Contains soluble chloride and fluoride, nitrate, etc.; and Hg, CdPb, Ni, As, Cr and other heavy metal ions. Based on these properties of wet desulfurisation wastewater, the main physical and chemical method for different types of pollutants, respectively, to create the corresponding physical and chemical reaction conditions, so that it is completely removed, the main reaction steps are as follows: (1) PH adjustment: calcium hydroxide/lime slurry [Ca (OH) 2] alkaline wastewater treatment, some of the heavy metals in the form of hydroxides precipitated, and neutralise the acidic substances in the wastewater. Adjust the wastewater PH value between 9.0 and 9.5 to create suitable reaction conditions for the subsequent treatment process. (2) Precipitation treatment: add organic sulfide, TMT (Trimer ~ Capto-Trianzin), so that certain heavy metals form precipitation such as cadmium, lead, mercury and so on. (3) Flocculation, coagulation treatment: through the addition of flocculant FeClSO4 and the appropriate amount of coagulant (polymerised electrolyte anionic) to make most of the suspended matter in the wastewater precipitation. (4) Pressing and neutralisation treatment: the precipitate is dewatered through a filter press, and hydrochloric acid is added to neutralise the discharge.
3 wastewater treatment equipment and process introduction
According to the process principle of desulphurisation wastewater treatment, desulphurisation wastewater treatment mainly includes the following four sub-systems: wastewater treatment system, chemical dosing system, sludge treatment system and supporting monitoring and automation system.
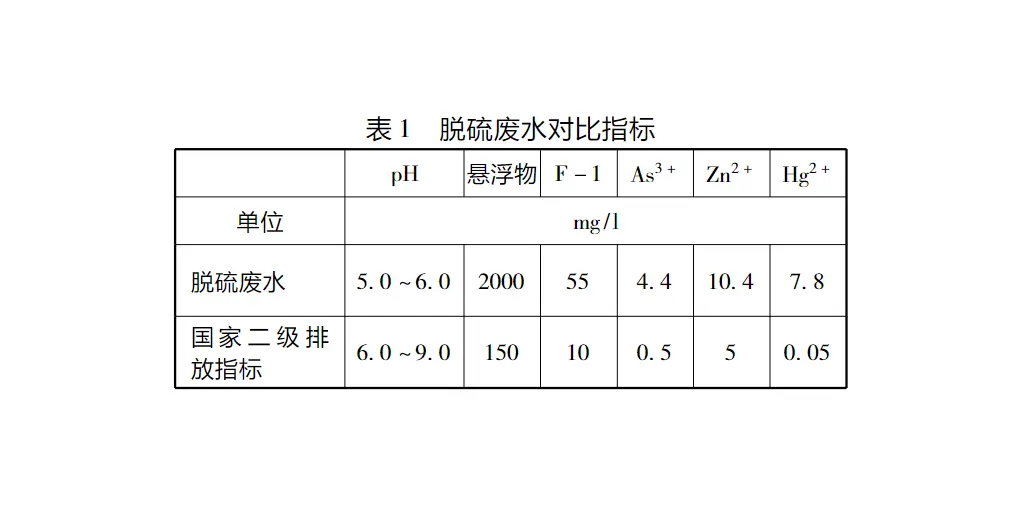
3.1 Wastewater treatment process (Figure 1)
(1) limestone alkalisation treatment, this step is mainly completed in the pH process adjustment box. Alkalisation treatment using calcium hydroxide/lime slurry [Ca(OH)2], plus lime slurry for wastewater alkalisation treatment, the acid in the water (H2SO4, H2SO3) according to the following reaction to be neutralised:H2SO4+Ca(OH)→2CaSO4+2H2OH2SO3+Ca(OH)→2CaSO3+2H2OGenerally trivalent heavy metal ions are more likely to be precipitated than bivalent Metal ions are easier to precipitate, when the PH value reaches 9.0 ~ 9.5, divalent and trivalent heavy metal ions through the formation of slightly soluble hydroxide precipitated from the wastewater, while the lime slurry Ca2+ can also react with some of the F- in the wastewater, generating insoluble CaF2; and complex with the As3+ to generate Ca (AsO2)2 and other insoluble substances. Reaction formula into the following:Me2++2OHMe(OH)2Me3++3OHMe(OH)3Trivalent metal ions precipitation of the PH value is usually lower than the divalent metal ions, due to the heavy metal ions to different PH value precipitation out of this step, so this step is the formation of each hydroxide of the decisive step. In addition, the pH value at which the precipitation of metal ions occurs is also affected by the presence of a large amount of excess electrolytes in the FGD wastewater.In the PH process adjustment box, the hydraulic retention time is about 1.0 h. Perforated aeration tubes are installed at the bottom of the tank to pre-aerate the FGD wastewater and increase the dissolved oxygen in the water. Because the desulphurisation wastewater is discharged at a high temperature, so the perforated stainless steel pipe. (2) precipitation treatment, this reaction step is mainly completed in the precipitation tank. Not all heavy metals can be completely precipitated out in the form of hydroxide, especially cadmium and mercury. Therefore, organic sulphur (TMT15) is added proportionally to the amount of wastewater being treated. Organic sulphur first forms slightly soluble compounds with cadmium and mercury, which are precipitated as solids. The basic reaction principle equation is (in the case of mercury):Hg+S-2HgS.(3) Flocculation and coagulation aid reaction:This stage is completed between the flocculation tank in the flocculation tank and the sedimentation and concentration tank:The hydroxides and sulphides that precipitate out of the settling tank are fine particles as are the solids in the original wastewater from the desulphurisation, dispersed throughout the system, and are very difficult to settle. In order to improve the settling behaviour of the solids, a flocculant (FeClSO4) was added to the wastewater to form an iron hydroxide/Fe(OH)3 small particle floc. Heavy metal hydroxides and compounds attach to the iron hydroxide small-particle flocs to form larger, more settleable flocs. This process is completed in the flocculation tank. The flocculation tanks are equipped with agitators to ensure uniform mixing of the wastewater and chemicals. In order to minimise fragmentation of the formed floc, the agitators in the flocculation tanks rotate at a slightly lower speed than in the first two reaction tanks. In order to promote the formation of flocculated particles in the settling tank and flocculation tank, it is necessary to add a small constant amount of contact slurry drawn from the clarifying and concentrating tank, which has formed the nuclei of the settled material, as shown in the FGD wastewater process flow diagram. Wastewater flows out of the flocculation box through the pipe mixer, that is, to which the coagulant aid (polyelectrolyte anionic type), in order to produce more easily settled large flocculated particles.
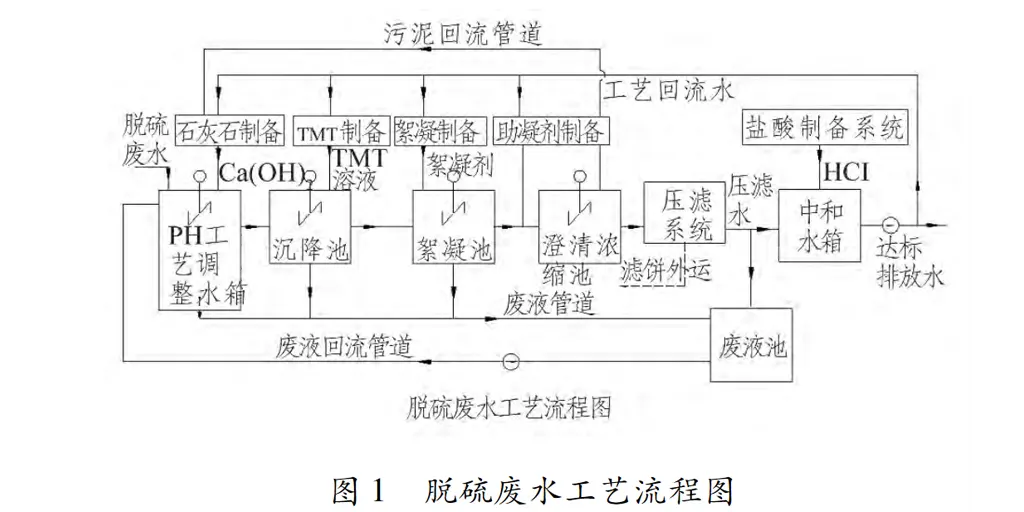
3.2 Chemical dosing system The role of the chemical system is mainly for the storage of drugs, stirring and mixing, formulated into the required concentration of the solution, are quantitatively added by the metering pump to the corresponding dosing point. Dosing system includes lime slurry dosing system, organic sulfur (TMT15) dosing system, flocculant (FeCLSO4) dosing system, coagulant dosing system, hydrochloric acid neutralisation dosing system. The dosage control indicators and other information of each system are shown in Table 2.
3.3 The relevant technical characteristics of the sludge press treatment system and filter press In order to completely remove all kinds of impurities generated in the FGD wastewater, the popular treatment programme at this stage is to use vacuum dehydration belt to vacuum filter the FGD saturated solution, or leaching. The use of vacuum belt filtration is prone to the following problems, which seriously affects the quality of desulfurisation wastewater and filtered solids: (1) the dehydration belt system supporting equipment is more than one, and the impact of system failure. Points are many. (2)Restricted by the operation of the desulphurisation system, such as gypsum quality is not high resulting in the quality of the dewatering belt dewatering and the quality of the solid output is not high. Plate and frame filter press in practical application, simple structure, compact equipment, large filtration area but small footprint, high operating pressure, low water content of the filter cake, strong applicability to a variety of materials, suitable for intermittent operation occasions.
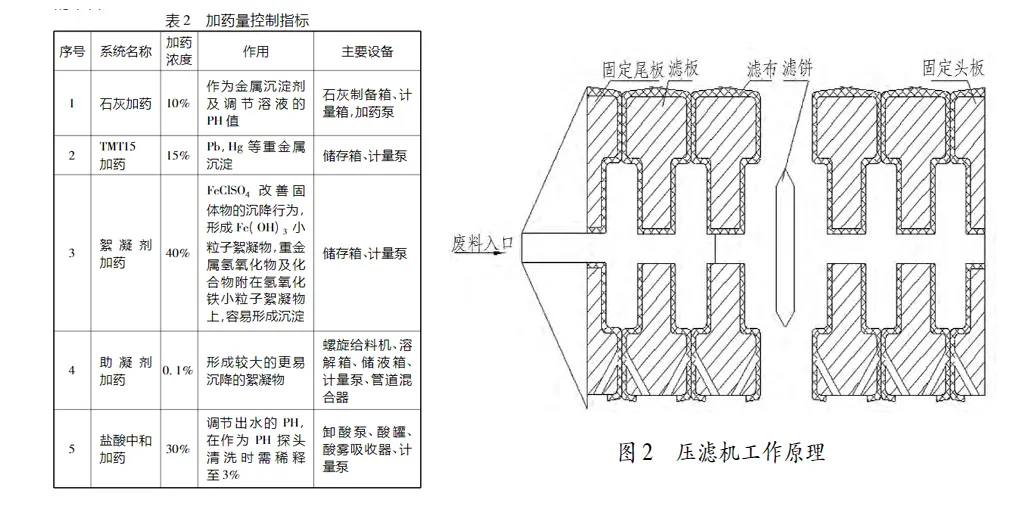
The filter chamber of the plate and frame filter press consists of filter plates and frames arranged in groups. The filter plate of the plate and frame filter press is designed with grooves on the surface for installing and supporting the filter cloth and guiding the flow direction of the filtrate, while the filter frame and the filter plate constitute the liquid circulation channel after assembly, which is used to pass into the suspension, washing water and lead out the filtrate. The filtrate through the feed pump under a certain pressure, from the fixed tail plate feed holes into the various filter chambers, through the filter cloth, the solids are retained in the filter chamber, and gradually formed into a filter cake; the liquid is discharged through the water outlet holes on the plate and frame. As the filtration process proceeds, the cake filtration begins, the thickness of the cake gradually increases, and the filtration resistance increases. The longer the filtration time, the higher the separation efficiency. The frame pressure filter press automatically completes the solid and liquid separation and other processes by pressing the filter plate, feeding, cake pressing, cake washing, cake blowing, discharging and other processes. The structure and working principle diagram is shown in Figure 2.
Welcome to call us for consultation, technical exchange, and material experiment.
Enquiry: 188517-18517
 Plate and frame chamber diaphragm filter presses
Plate and frame chamber diaphragm filter presses